-
 Introduction to Organic Chemistry 9th class Chemistry
Introduction to Organic Chemistry 9th class Chemistry

When methane was mentioned at the end of Section 4.2 "Covalent Compounds: Formulas and Names", we described it as the simplest organic compound. In this section, we introduce organic chemistry more formally.
Organic chemistry is the study of the chemistry of carbon compounds. Carbon is singled out because it has a chemical diversity unrivaled by any other chemical element. Its diversity is based on the following:
- Carbon atoms bond reasonably strongly with other carbon atoms.
- Carbon atoms bond reasonably strongly with atoms of other elements.
- Carbon atoms make a large number of covalent bonds (four).
Curiously, elemental carbon is not particularly abundant. It does not even appear in the list of the most common elements in Earth’s crust. (See Table 2.1 "Elemental Composition of Earth" in Chapter 2 "Elements, Atoms, and the Periodic Table".) Nevertheless, all living things consist of organic compounds.
Most organic chemicals are covalent compounds, which is why we introduce organic chemistry here. By convention, compounds containing carbonate ions and bicarbonate ions, as well as carbon dioxide and carbon monoxide, are not considered part of organic chemistry, even though they contain carbon.
The simplest organic compounds are the hydrocarbons, compounds composed of carbon and hydrogen atoms only. Some hydrocarbons have only single bonds and appear as a chain (which can be a straight chain or can have branches) of carbon atoms also bonded to hydrogen atoms. These hydrocarbons are called alkanes (saturated hydrocarbons). Each alkane has a characteristic, systematic name depending on the number of carbon atoms in the molecule. These names consist of a stem that indicates the number of carbon atoms in the chain plus the ending -ane. The stem meth- means one carbon atom, so methane is an alkane with one carbon atom. Similarly, the stem eth- means two carbon atoms; ethane is an alkane with two carbon atoms. Continuing, the stem prop- means three carbon atoms, so propane is an alkane with three carbon atoms. Figure 4.6 "Formulas and Molecular Models of the Three Simplest Alkanes" gives the formulas and the molecular models of the three simplest alkanes. (For more information about alkanes, see Chapter 12 "Organic Chemistry: Alkanes and Halogenated Hydrocarbons".)
Figure 4.6 Formulas and Molecular Models of the Three Simplest Alkanes
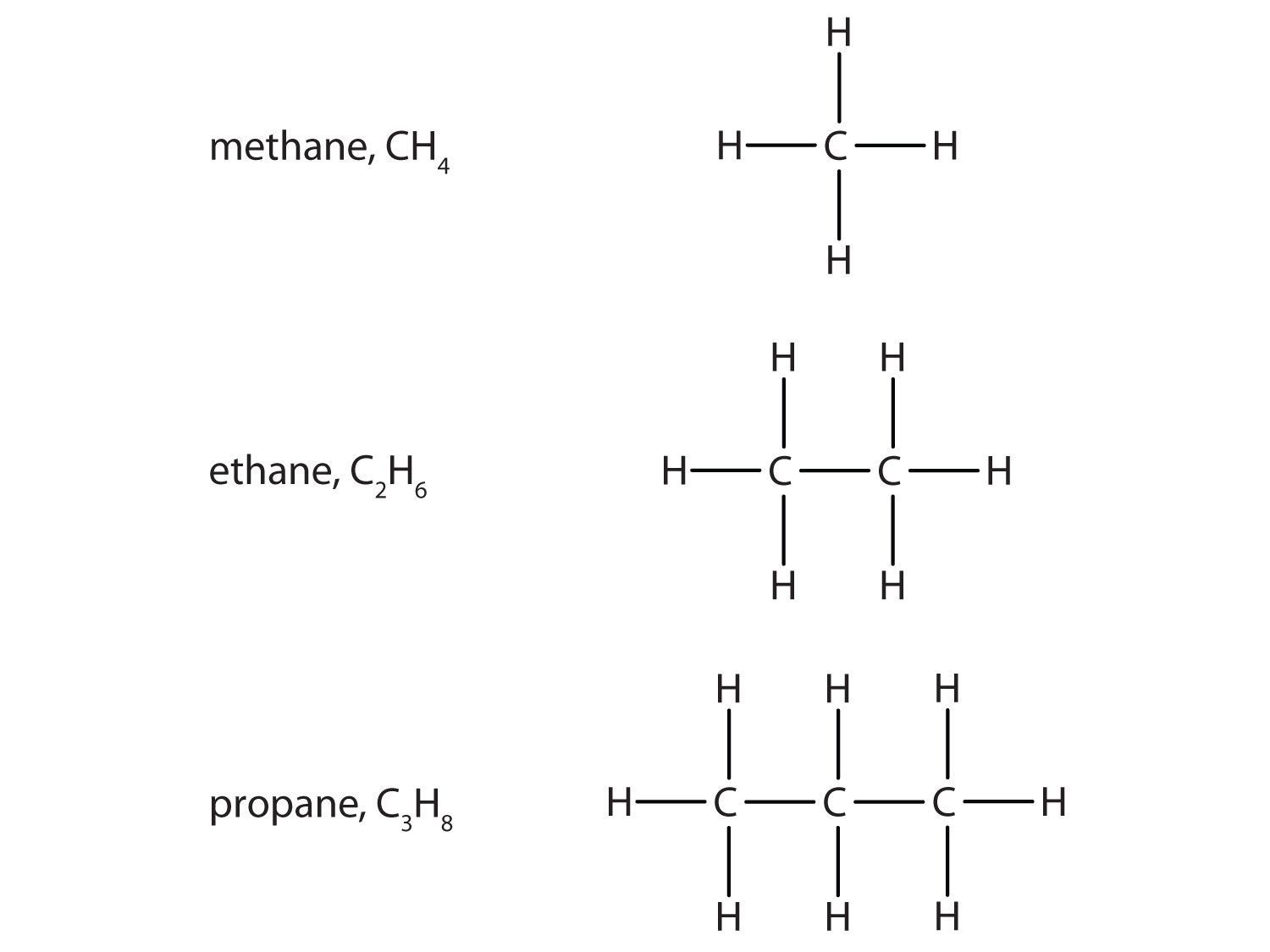
The three smallest alkanes are methane, ethane, and propane.
Some hydrocarbons have one or more carbon–carbon double bonds (denoted C=C). These hydrocarbons are called alkenes. Figure 4.7 "Formulas and Molecular Models of the Two Simplest Alkenes" shows the formulas and the molecular models of the two simplest alkenes. Note that the names of alkenes have the same stem as the alkane with the same number of carbon atoms in its chain but have the ending -ene. Thus, ethene is an alkene with two carbon atoms per molecule, and propene is a compound with three carbon atoms and one double bond.
Figure 4.7 Formulas and Molecular Models of the Two Simplest Alkenes
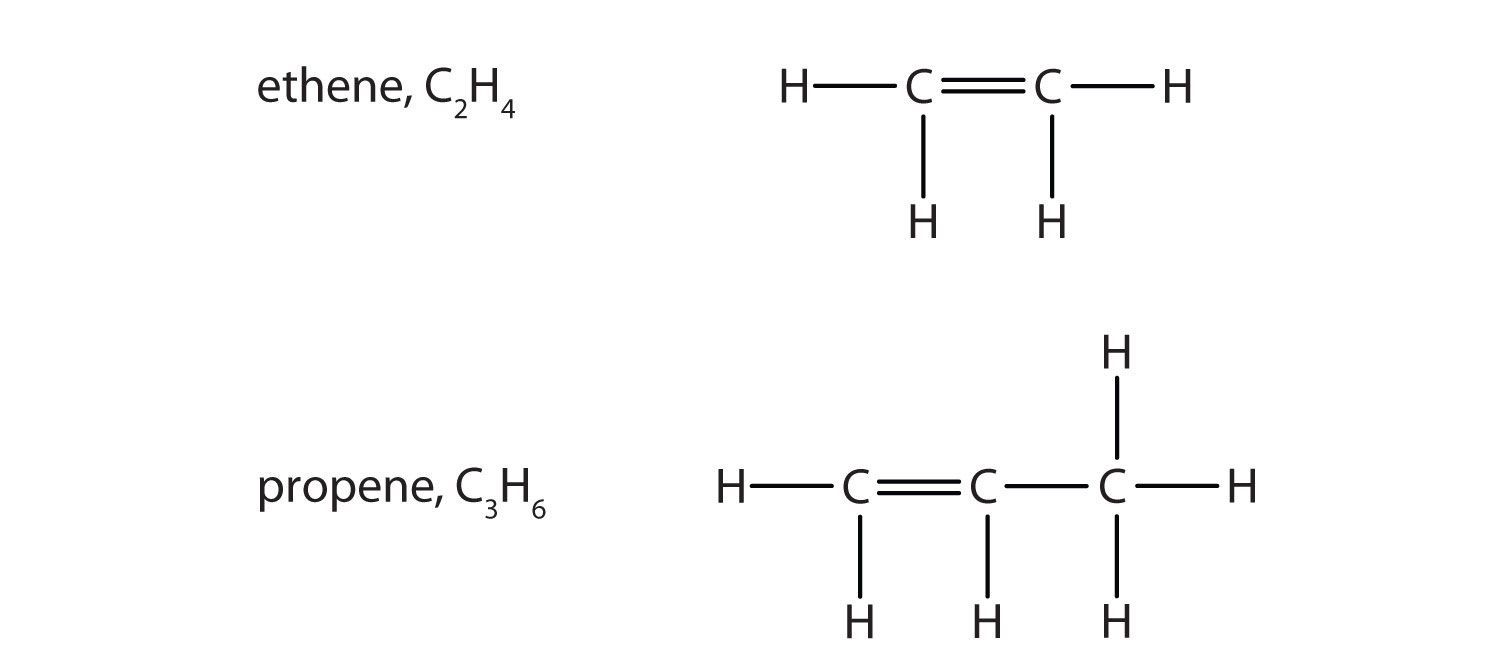
Ethene is commonly called ethylene, while propene is commonly called propylene.
Alkynes are hydrocarbons with a carbon–carbon triple bond (denoted C≡C) as part of their carbon skeleton. Figure 4.8 "Formulas and Molecular Models of the Two Simplest Alkynes" shows the formulas and the molecular models of the two simplest alkynes and their systematic names. The names for alkynes have the same stems as for alkanes but with the ending -yne. (For more information about alkenes and alkynes, see Chapter 13 "Unsaturated and Aromatic Hydrocarbons".)
Figure 4.8 Formulas and Molecular Models of the Two Simplest Alkynes
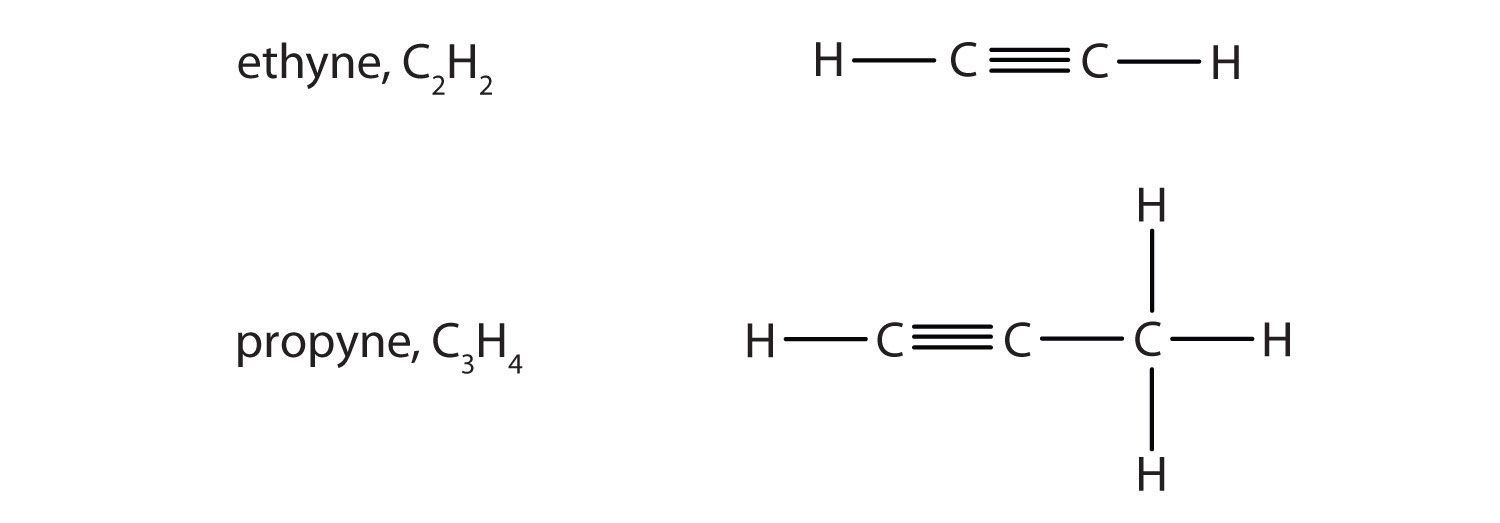
 Posting Permissions
Posting Permissions
- You may not post new threads
- You may not post replies
- You may not post attachments
- You may not edit your posts
-
Forum Rules









 Reply With Quote
Reply With Quote